Why vanadium batteries are becoming more and more popular
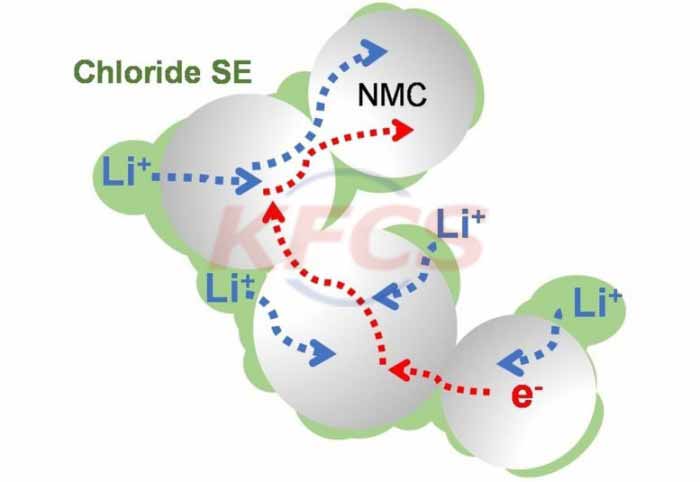
The full name of vanadium battery is all vanadium redox flow battery. The principle is to use vanadium oxide to contact with the electrode to generate electrochemical reaction, so that chemical energy can be converted into electrical energy. The vanadium ion electrolyte contains chemical energy, which is stored in the storage tank by the vanadium battery energy storage system. The external pump is used to press the electrolyte from the storage tank into the battery stack, so that the electrolyte flows through the electrode surface in parallel and generates electrochemical reaction, thereby completing the charging or discharging of the vanadium battery energy storage system.
First of all, the vanadium oxide needed by the vanadium battery can exist in the aqueous solution, so it is not easy to have thermal runaway, combustion and explosion; However, lithium has good chemical activity, is unstable in water, can only exist in organic electrolyte, and is flammable and explosive;
Second, the electrolyte of the vanadium battery is separated from the stack, so the phenomenon similar to the spontaneous combustion of the lithium battery due to the internal short circuit will not occur;
Third, when dealing with the demand of medium and large-scale energy storage, the vanadium battery system only needs to increase the volume of electrolyte, which is safe and controllable; However, the number of lithium batteries needs to be increased, and the greater the number, the greater the probability of accidents. Moreover, a large number of lithium batteries are stored in a centralized manner, which is more likely to be dangerous.