Vanadium redox battery suitable for high power energy storage
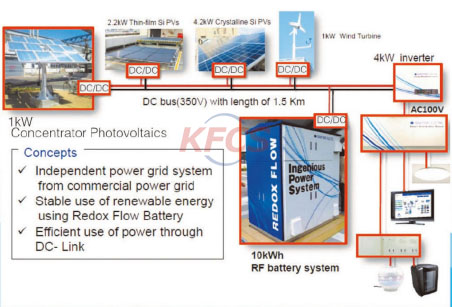
Vanadium redox battery suitable for high power energy storage In order to alleviate the energy crisis and environmental pressure, we need to vigorously develop and utilize renewable energy such as wind energy and solar energy. However, wind energy and solar energy are greatly affected by the affected areas, seasons and climates, and their power generation is discontinuous and stable, so it is necessary to use energy storage devices to store power when there is excess power. In the case of power shortage, grid-connected power generation can be stored, thus realizing stable and continuous power output. Comprehensive evaluation based on efficiency, cost and flexibility; Among the energy storage systems, electrochemical energy storage system has the best performance at present. However, lithium battery is a common electrochemical energy storage device, which has the characteristics of high cost and high temperature resistance, and is only suitable for small or micro mobile power supply.
Another lead-acid battery is a common electrochemical energy storage device, which has the advantages of low energy density and long service life: short service life, rapid capacity reduction after repeated charge and discharge, and easy to cause environmental pollution. They are not suitable for high-power energy storage devices such as power plants. In order to meet the needs of social development, people are developing new high-tech energy storage batteries, among which redox flow battery is a research hotspot in this field. This paper introduces a promising vanadium redox flow battery; In the period of low power consumption, the VRF battery, which is generated by solar energy or wind energy, is charged, and the electric energy is converted into chemical energy for storage. The essence of this process is to apply direct current, forcing the spontaneous oxidation-reduction reaction to take place in reverse. During peak power consumption, the battery discharges. Material changes in the discharge process and external circuit electrons V2+ spontaneously lose electrons on the surface of the negative electrode material and turn into V3, which constitutes the negative reaction of the battery. A spontaneous redox reaction takes place inside the battery to realize the conversion of chemical energy and electric energy.