Research: Breakthrough in cathode chemistry paves way for more sustainable lithium-sulfur batteries
Researchers at Drexel University have developed a stable sulfur cathode that can run thousands of times in the carbonate electrolyte used in commercial lithium-ion batteries, paving the way for more sustainable battery replacements.
Growing demand for electric vehicles (EVs) in the U.S. has revealed significant challenges in sustainably sourcing battery technology necessary for a broad shift toward renewable electricity and away from fossil fuels. To make batteries not only perform better than those currently used in electric vehicles, but also be made from off-the-shelf materials, a team of chemical engineers at Drexel University has found a way to introduce sulfur into lithium-ion batteries -- results surprising.
As global EV sales double in 2021, prices for battery materials such as lithium, nickel, manganese and cobalt have soared, and supply chains for these raw materials, mostly from other countries, have also been bottlenecked by the pandemic. This also focuses attention on the main suppliers of raw materials: countries such as Congo; and raises the question of the human and environmental impact of extracting these raw materials from the earth.
Developing a commercially viable sulfur battery has been a sustainable, high-performance goal for the battery industry long before the proliferation of electric vehicles and a shortage of battery materials. This is because the natural abundance and chemical structure of sulfur will allow it to store more energy. A recent breakthrough, published in the journal Communications Chemistry, by researchers at Drexel University's School of Engineering offers a way to circumvent the hurdles that have held down lithium-sulfur batteries in the past, finally bringing the highly sought-after technology to the fore. to the scope of commercialization.
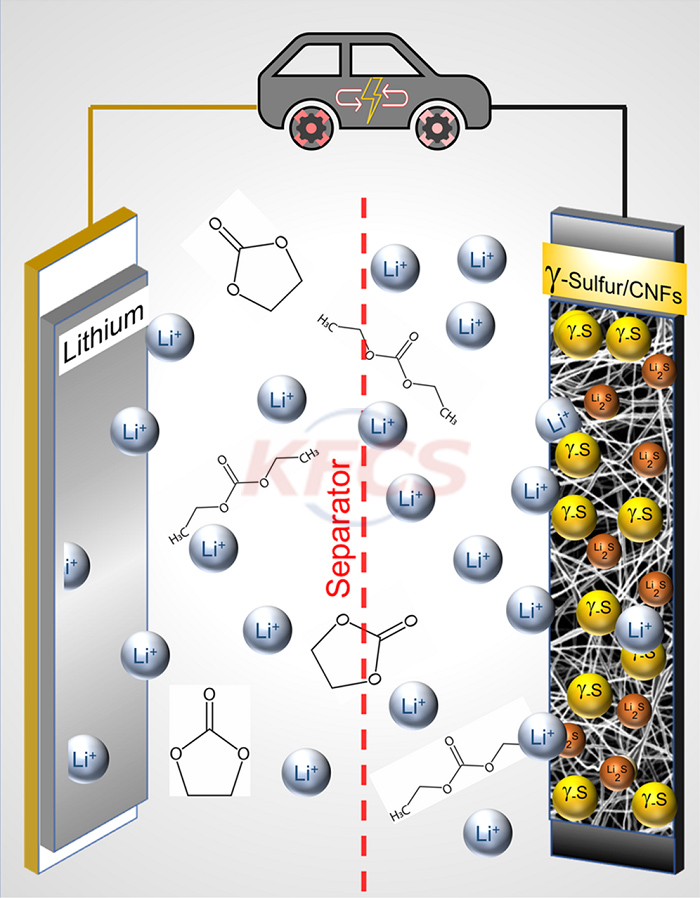
Their discovery is a new way to produce and stabilize a rare form of sulfur that functions in carbonate electrolytes -- the energy-transfer liquids used in commercial lithium-ion batteries. Not only will this development make sulfur batteries commercially viable, but they will have three times the capacity of lithium-ion batteries and last for more than 4,000 charges -- equivalent to 10 years of use, which is also a substantial improvement.
Dr Vibha Kalra, the George-B-Francis Professor of Chemical and Biological Engineering at Drexel University, who led the research, said: "Sulfur has been ideal for use in batteries for many years because it is an abundant resource on Earth, It can be collected in a safe and environmentally friendly manner, and as we have now demonstrated, it also has the potential to improve battery performance in electric vehicles and mobile devices in a commercially viable manner."
The challenge of introducing sulfur into lithium batteries with commercially friendly carbonate electrolytes is the irreversible chemical reaction between the intermediate sulfur products, known as polysulfides, and the carbonate electrolyte. Due to this undesirable reaction, previous attempts to use a sulfur cathode in a battery with a carbonate electrolyte solution have resulted in an almost immediate shutdown and complete failure after just one cycle.
Lithium-sulfur (Li-S) batteries have already demonstrated superior performance in experimental settings using ether electrolytes -- rather than carbonates -- because ether does not react with polysulfides. But these batteries are not commercially viable because the ether electrolyte is highly volatile, and its components have a boiling point as low as 42 degrees Celsius, meaning that any battery heating above room temperature could lead to failure or melting.
"In the past decade, most lithium-sulfur fields have adopted ether electrolytes to avoid adverse reactions with carbonates," Kalra said. "Then over the years, researchers have delved into how to mitigate so-called polysulfide shuttling/diffusion. Improving the performance of ether-based sulfur batteries -- but the field completely ignores the fact that ether electrolytes are themselves a problem. In our work, the primary goal was to replace ether with carbonates, but in doing so, we Polysulfides are also eliminated, which also means there is no shuttle, so the battery can perform exceptionally well for thousands of cycles."
Previous research by Kalra's team has also approached the problem this way -- producing a carbon nanofiber cathode that slows the shuttle effect in ether-based lithium-sulfur batteries by curbing the movement of intermediate polysulfides. But to improve the commercial pathway for cathodes, the team realized it needed to make them work with a commercially viable electrolyte.
"Having a cathode that works with the carbonate electrolytes they already use is the path of least resistance for commercial manufacturers," Kalra said. "So our goal is not to drive industry adoption of a new electrolyte, but is to make a cathode that can work in existing lithium-ion electrolyte systems."
So, in hopes of eliminating polysulfide formation to avoid adverse reactions, the team attempted to confine sulfur in the carbon nanofiber cathode substrate using evaporation techniques. While the process didn't succeed in embedding sulfur into the nanofiber web, it did something extraordinary, which became apparent when the team started testing the cathode.
"When we started testing it started working beautifully - something we didn't expect. In fact, we tested it over and over - over 100 times - to make sure we were really seeing what we thought we were seeing. stuff," Kalra said. "We suspected that the sulfur cathode would cause the reaction to stall, but it actually performed surprisingly well, and it did so over and over again without causing a shuttle."
Upon further investigation, the team found that during the process of depositing sulfur on the surface of carbon nanofibers -- changing it from a gas to a solid -- it crystallized in an unexpected way, creating a slight variation of the element , known as monoclinic gamma phase sulfur. This chemical phase of sulfur, which does not react with carbonate electrolytes, has previously only been produced at high temperatures in the laboratory and has only been observed in the extreme environments of natural oil wells.
Rahul Pai, a PhD student in the Department of Chemical and Biological Engineering and co-author of the study, said: "At first, it was hard to believe that this is what we detected, because in all previous studies, monoclinic gamma phase sulfur was below 95 degrees Celsius. is unstable. In the last century, only a few studies have produced monoclinic gamma phase sulfur, and it was only stable for 20-30 minutes at most. But we created it in a cathode that went through several Thousands of charge-discharge cycles with no loss of performance -- our inspection of it a year later showed that the chemical phase has remained the same."
After more than a year of testing, the sulfur cathode remained stable, with no degradation in performance over 4,000 charge-discharge cycles, equivalent to 10 years of regular use, as the team reported. And, as predicted, the battery has more than three times the capacity of a lithium-ion battery.
"While we are still working to understand the exact mechanism that creates this stable single-crystal sulfur at room temperature, this is still an exciting discovery that could lead to the development of more sustainable and affordable battery technologies," Kalra said. Open many doors."
Replacing cathodes in lithium-ion batteries with sulfur would ease the need to procure cobalt, nickel and manganese. The supply of these raw materials is limited and not easy to extract, causing no health and environmental hazards. On the other hand, there is sulfur all over the world, and there is a lot of it in the US because it's a waste product from oil production.
Kalra suggested that having a stable sulfur cathode, functioning in a carbonate electrolyte, would also allow researchers to make progress in researching alternatives to lithium anodes -- which could include more earthly resource options such as sodium.
Kalra said: "Weaning away from reliance on lithium and other materials that are expensive and difficult to extract from the earth is a crucial step for the development of batteries and for expanding our ability to use renewable energy. The development of a viable lithium-sulfur Batteries open up many avenues for replacing these materials."